Fuel cells are electrochemical energy conversion devices that cleanly and efficiently produce electricity by combining hydrogen and oxygen into water. Like batteries, fuel cells convert potential chemical energy into electrical energy and generate heat as a by-product.
While chemical energy is stored inside batteries, fuel cells can continuously generate electricity as long as they are supplied with fuel and oxygen. While the first fuel cells were introduced in 1838, it wasn’t till a century later that they were tapped for commercial use following the invention of the hydrogen-oxygen fuel cell in 1932.
Since then, fuel cells have been used in NASA space programs to generate power for satellites and space capsules and as primary and backup power systems for commercial, industrial and residential buildings and in remote or inaccessible areas. They are also used to power fuel cell vehicles, including forklifts, automobiles, buses, trains, boats, motorcycles and submarines.
Fuel cell design
Fuel cells come in many different varieties; however they all function similarly and are made of three adjacent components – an anode, electrolyte and cathode. The electrolyte usually defines the type of fuel cell and can be made from many substances, including potassium hydroxide, salt carbonates, or phosphoric acid. Fuel cells designed for vehicles will typically feature a polymer electrolyte membrane (PEM) that uses a layered material that looks similar to plastic wrap, which conducts only positively charged ions and blocks electrons.
The anode catalyst is usually made of fine platinum powder, which breaks down the fuel into electrons and ions. In contrast, the cathode catalyst, often nickel, converts ions into waste chemicals such as water. A catalyst layer is added on both sides of the PEM membrane, with the anode layer on one side and the cathode layer on the other.
The layers include nanometer-sized particles of platinum dispersed on a high-surface-area carbon support. This supported platinum catalyst is mixed with an ion-conducting polymer and sandwiched between the membrane and gas diffusion layers (GDLs). On the anode side, the platinum catalyst enables hydrogen molecules to be split into protons and electrons. On the cathode side, the platinum catalyst enables oxygen reduction by reacting with the protons generated by the anode, producing water. The ionomer mixed into the catalyst layers allows the protons to pass through those layers.
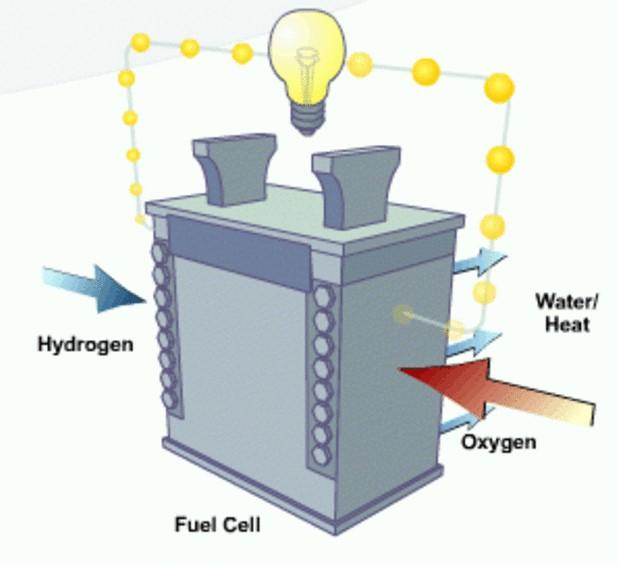
Fig 1. A fuel cell functions similar to a battery and uses the energy created from molecules passing from an anode to the cathode to generate power. (Credit: Department of Energy)
How fuel cells function
As mentioned above, fuel cells function similarly to batteries but do not run down or require recharging. Most cells feature two electrodes - a negative electrode (or anode) and a positive electrode (or cathode), sandwiched around an electrolyte. (Fig 1.) A fuel, such as hydrogen, is introduced to the anode, while air is fed to the cathode. In a hydrogen fuel cell, a catalyst at the anode separates hydrogen molecules into protons and electrons, which take different paths to the cathode. The electrons pass through an external circuit, creating a flow of electricity, then migrate through the electrolyte to the cathode, where they combine with oxygen and the electrons to produce water and heat.
The electrolyte in the cell plays a key role, as it must permit only the appropriate ions to pass between the anode and cathode. If free electrons or other substances manage to travel through the electrolyte, they will disrupt the chemical reaction. Whether they combine at the anode or cathode, the hydrogen and oxygen will produce water, which drains from the cell. So, as long as a fuel cell is supplied with hydrogen and oxygen, it will generate electricity.
Since fuel cells create electricity via chemical reaction, and not by combustion, they are not subject to the thermodynamic laws limiting a conventional power plant. Thus, fuel cells are more efficient in extracting energy from fuel, and the waste heat generated from some cells could also be harnessed via a thermoelectric generator, boosting system efficiency even further.
Types of fuel cells
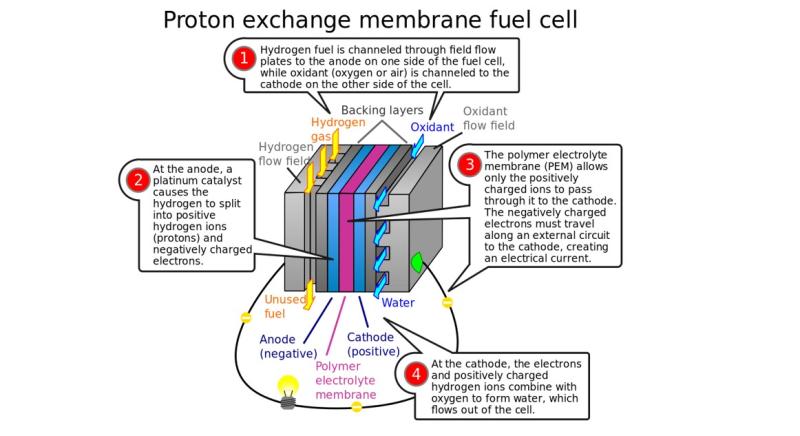
Fig 2. PEM-based fuel cells take advantage of hydrogen as the fuel source and use a solid polymer as the electrolyte. (Credit: Jafet via Pixabay)
Proton exchange membrane fuel cells
Many types of fuel cells power everything from vehicles to industrial manufacturing plants. One of the more commonly used is the proton exchange membrane fuel cell, where the hydrogen diffuses to the anode catalyst and later dissociates into protons and electrons. (Fig 2.)The protons react with oxidants causing them to become what is known as multi-facilitated proton membranes. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit because the membrane is electrically insulating. Oxygen molecules react with the electrons and protons on the cathode catalyst to produce water.
Alkaline fuel cells
Alkaline fuel cells (AFCs) were adopted by NASA to provide power and drinkable water in space, where resources are a critical commodity. These fuel cells use a solution of potassium hydroxide in water as the electrolyte and can employ a variety of non-precious metals as a catalyst at the anode and cathode. They are also closely related to conventional PEM fuel cells, except they use an alkaline membrane instead of an acid membrane. AFCs are considered high-performance cells because of the rate at which electrochemical reactions occur in the cell. They have also demonstrated efficiencies above 60% in space applications.
Solid oxide fuel cells
Solid oxide fuel cells (SOFCs) take advantage of solid material, most commonly a ceramic material known as yttria-stabilized zirconia (YSZ), as the electrolyte. Because SOFCs are manufactured entirely of solid materials, they are not limited to the layered configuration of other types of fuel cells and are often designed as rolled tubes. These types require high operating temperatures (800 to 1000°C) and can be run on various fuels, including natural gas. Those high temperatures also remove the need for a precious-metal catalyst and allow the cells to reform fuels internally, enabling the use of different fuels.
This is just an introduction to fuel cells, how they function and the materials that make up their design. As with any energy source, they have their benefits and drawbacks, which are slowly being mitigated as new technologies become available.