Perovskites are a class of materials with great promise in electronics, thanks to their wide array of tuneable dielectric* (insulator) properties. However, these properties hinge on the presence or absence of inherent structural defects and impurities, as well as on environmental conditions such as temperature. Understanding these influences is critical for developing better-performing electronic components. Here is a summary of how temperature-controlled analysis can be used to bring out these properties.
Investigating novel material responses to environmental conditions
Over the last decade, perovskite-based materials have emerged as promising materials for a wide range of electronic devices, including photovoltaics, piezoelectrics, optoelectronics and superconductors. CaTiO3 (calcium titanate) is the archetypal perovskite, but many others (with the same basic ABX3 formula) are possible thanks to the ability of a wide variety of metal ions to take the A and B positions, and the search is constantly on for more systems with better properties. (especially those that don’t contain lead, on account of its toxicity.
Defects and surface interactions are well-known to affect the behavior of perovskites, making it vital to understand their properties in detail. Spectroscopic techniques, as well as conventional electrical probes, are widely used, but a challenge is doing so under controlled conditions of temperature. Below are two examples of how temperature-controlled stages such as those in Figure 1 have been used to uncover insights into perovskite performance.

Figure 1: Example of a device suitable for high-temperature studies, allowing control of temperature up to 1500°C, and with electrical connections allowing measurement during heating experiments. The sample is placed inside the ceramic sample cup, enabling it to be heated from underneath as well as from the sides, at rates of up to 200°C/min.
Investigating defects in LaAlO3
Among the many perovskites under study, LaAlO3 (lanthanum aluminate) is top of the list for thin-film applications, because its relatively high dielectric constant makes it a good insulator. The properties of the pure compound are unexceptional, but interesting properties such as magnetism and superconductivity can emerge when small amounts of impurities are present, or at the interface with other perovskites, such SrTiO3 (strontium titanate).
To make sense of these interactions, the properties of pure LaAlO3 first need to be understood. A study led by Surajit Saha at the National University of Singapore used Raman spectroscopy to study the vibrational modes of the lattice in pure LaAlO3 crystals, using a thermal stage to see how the spectra varied with temperature, from –193°C to 26°C.
The group found that many of the signals in the Raman spectra ‘split’ when a strong magnetic field was applied, with some (such as those marked P1–P3 in Figure 2) splitting at a magnetic field of 2 T, whereas others (such as those marked P16–P17) splitting only at 4 T. All the splitting’s, and indeed many of the signals themselves, disappeared above about –170°C.
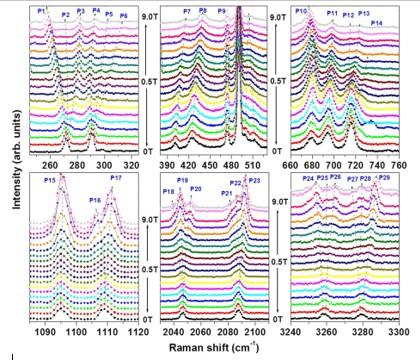
Figure 2: Raman spectra of LaAlO3 at different wavelengths, showing splitting of the peaks at 271 and 290 cm−1 at 2 T (top left, P1–P6) and splitting of peaks at 1094 and 1108 cm−1 at 4 T (bottom left).
As a result of these observations, they concluded that two different types of defects were present: for example, peaks P1–P3 could be due to mis-located oxygen atoms, whereas P16–P17 may involve ‘anti-sites’ (where the metal ions swap places), or simply the absence of metal ions. In both cases, the fact that splitting occurs at all indicates that the La ions must align in a plane within the crystal – which the authors say should help to understand how these defects could be engineered to produce new properties.
Studying the effect of additives in perovskites
BiFeO3 (bimuth ferrite) is a perovskite that holds promise because of its good ferroelectric and ferromagnetic properties even at high temperatures. However, pure BiFeO3 shows poor electrical insulation, so it is commonly combined with other perovskites. Amongst these, BiFeO3–BaTiO3 mixtures appear promising, but little was known about their ferroelectric and piezoelectric properties. A group led by Xiaochun Wu at Sichuan Normal University, Chengdu, China, aimed to resolve this by doping BiFeO3–BaTiO3 with MnO2 (magnesium dioxide) and Co2O3 (cobolt oxide), to give what is termed a ‘multiferroic’ perovskite.
They found that adding 1 mol% of both MnO2 and Co2O3 resulted in enhanced piezoelectricity, good ferroelectricity, and a higher depolarization temperature. To understand how the relative dielectric constant (otherwise known as relative permittivity) varied with temperature, an LCR meter was used in conjunction with a temperature-controlled probe stage. This allowed them to check that the material displayed good temperature stability, and as a result, they were able to conclude that this doped perovskite is a promising candidate for high-temperature applications.
The importance of temperature-controlled measurements
As demonstrated by these two examples, the easy acquisition of temperature-dependent measurements allows perovskites to be examined in greater depth, enabling researchers to identify promising materials for electronics applications. However, the options extend much further than merely temperature control – stages are also available for investigating the effect of gas purging, or under conditions of controlled vacuum or relative humidity.
With these tools available, electronics design and development engineers will be in a better position than ever before to maximize the insights obtained from spectroscopic and electronic measurements, and so develop new materials for applications in the electronics and semiconductor sectors.
*Dielectric: An insulating material, or very poor conductor of electrical current. These materials do not have free electrons in the way metals do, so electrical current cannot flow through easily.
Clara Ko is sales and marketing manager for UK-based Linkam Scientific Instruments.