Hydrogen has the potential to be an important source of clean fuel in our energy-driven economy. The use of hydrogen (H2) is growing rapidly in both traditional applications, such as petroleum refineries, and in newer sectors such as fuel cells and power generation. Based on recent market reports, global hydrogen consumption has grown from 21 million metric tons (Mmt) in 2005 to more than 32 Mmt in 2007. Global hydrogen usage is expected to surpass 50 Mmt by 2012 and is expected to exceed 79 Mmt by 2016. Currently, more than 90% of all hydrogen produced is used in the petroleum refining industry, where continuous monitoring of hydrogen is highly desired to improve both the quality and yield of hydrocarbon-based fuels. Because of hydrogen's combustible and explosive properties, its accurate detection is important for safe hydrogen transport, storage, and use.
Hydrogen Basics
Hydrogen is a flammable gas when combined with air in concentrations between 4% by volume (lower flammable limit) and 75% by volume (upper flammable limit). It is one of the least flammable materials at a concentration of 4% but has a larger window of flammability in comparison to natural gas, gasoline, propane, ethane, methane, and propylene. In fact, the flammability limit of hydrogen is seven times wider than that of methane (Figure 1). For safety applications, it is therefore critical that a hydrogen sensor have a wider measurement range (1%–99% v/v H2) than is required for most common fuels. Because hydrogen is the lightest of elements and the smallest molecule it has the greatest tendency to leak. In terms of process safety, a hydrogen leak can be more dangerous and harder to detect than leaks of other gases, because of its wider flammability and fast diffusion rate.
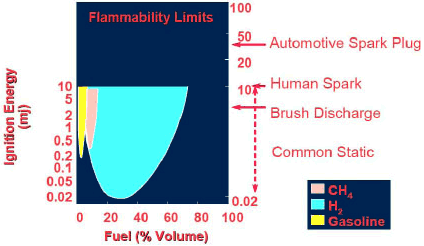 Figure 1. Schematic showing the flammability limits of hydrogen in comparison to gasoline and methane (CH4) |
Hydrogen Sensors, Operation, and Drawbacks
Any hydrogen sensor technology needs to satisfy the three basic requirements—sensitivity, selectivity, and specificity. It should be functional over the range of interest and be free of cross-interference from other gases present in the environment. Sensing technologies for hydrogen have been studied since the early 1900s and several traditional sensing mechanisms are still widely used in the industry: gas chromatography, mass spectrometry, catalytic bead, and thermal conductivity.
Solid-state metal oxide and catalytic bead sensors use heated catalysts to sense hydrogen. They require heating to around 300°C to enable surface reactions that promote hydrogen sensing and are not hydrogen-specific. Electrochemical sensors are based on known electrolytic reactions of hydrogen and use liquid or solid electrolytes. Sensors that use liquid electrolytes tend to have limited lifetimes for several reasons: they use a consumable (the electrolyte), their membranes have limited life spans, and they can have leakage issues.
The hydrogen sensors based on thermal conductivity, catalytic bead, metal oxide, and electrochemical technologies require the presence of oxygen for sensor operation. Oxygen plays a crucial role in promoting grain boundary formation in metal oxide sensors and electron transfer reactions in electrochemical sensors. In contrast, sensors that use palladium (Pd), a hydrogen-specific material, do not require oxygen for operation. Palladium-based sensors are gaining popularity in the industry because of their reliability and high specificity to hydrogen.
A Novel Hydrogen Sensor
The hydrogen-specific sensor technology developed by H2scan is based on the unique interaction between hydrogen and palladium. Molecular hydrogen dissociatively adsorbs on palladium surfaces and atomic hydrogen readily diffuses into the palladium lattice in a quantity proportional to the hydrogen partial pressure above the surface (Figure 2). H2scan hydrogen-specific sensors have two components that measure hydrogen atoms that have diffused into the palladium lattice:
- A metal insulator semiconductor (MIS) device (e.g., capacitor, FET, or Schottky diode) that detects hydrogen atoms as a charge at the interface of palladium and an underlying dielectric material
- A thin-film resistor whose resistance increases with increasing hydrogen uptake as the hydrogen atoms distort the palladium lattice and increase the number of scattering sites for electrons.
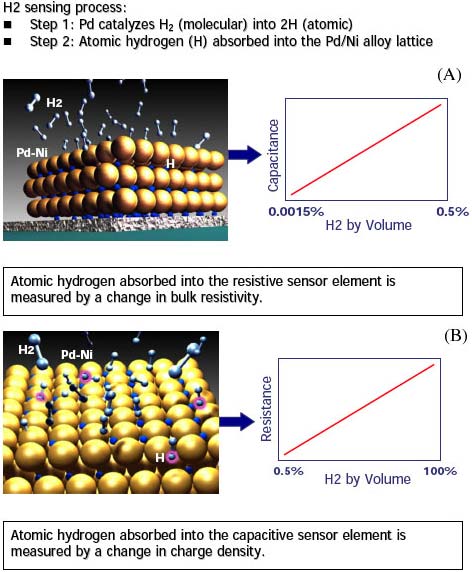 Figure 2. Schematic of the operation of H2Scan's hydrogen-specific (A) capacitor and (B) resistor |
The MIS device (a capacitor in current products) is sensitive to hydrogen concentrations from a few ppm to about 0.5% v/v (Figure 2A), while the thin-film resistor is sensitive from concentrations of hundreds to thousands of parts per million (depending on background gases) to 100% hydrogen (Figure 2B) at several atmospheres. Because the sensor responds only to hydrogen in the palladium lattice, the sensors are fundamentally specific to hydrogen and this creates several advantages over existing technologies. For example, H2scan sensors can operate in inert environments because they do not require oxygen to operate and they do not require a premixed reference gas or precise control of analysis gas temperature as thermal conductivity analyzers do. Furthermore, because only the partial pressure of atomic hydrogen is directly measured, the sensing mechanism is fundamentally specific to hydrogen.
In addition to the hydrogen-sensitive MIS and resistor devices, the H2scan sensor has integrated temperature control elements—a temperature measurement device and a resistive heater (Figure 3)—created using the company's "chip on a flex" technology. This independent temperature control allows the sensor to tolerate a wide range of gas temperature and flow conditions.
 Figure 3. The sensor in the company's patented "chip on a flex" platform. The sensor chip (3 mm x 4 mm) contains the hydrogen-sensitive resistor and capacitor, an on-chip temperature sensor, and a heater |
The company has also developed a proprietary protective barrier. The molecular-level coating is deposited directly on the sensor surface where it allows the sensor to operate in the presence of carbon monoxide (CO) and hydrogen sulfide (H2S) contaminants that would poison an unprotected palladium surface. The sensor system can tolerate continuous operation in gas streams containing up to 100 ppm of CO and 1000 ppm of H2S. The hydrogen-specific analyzer has both a fine wire mesh and an automotive-grade GORE-TEX membrane separating the sensor from the analysis gas. These protect the sensor from particulates and from liquid droplets in mixed-phase gas streams, respectively. The hydrogen specificity provided by this design enables hydrogen leak detection, accurate hydrogen monitoring for safe area operation, and continuous inline process monitoring.
The process-hardened device can be installed at multiple points in a process plant and directly linked to a DCS or PLC. The Model 700 HY-OPTIMA, for example, is an online process monitor that has been tested in gas streams of 1000 ppm H2S, 100 ppm CO for refinery applications as well as in gas streams of 50% Cl2, 95% RH for corrosive applications. The ruggedness of the sensor extends its operational life in most process streams, including complex hydrocarbons, to more than 3 years, with low sensor replacement costs. Its robust, corrosion-resistant sensor probe can be installed into a gas stream with temperatures from –20°C to 100°C and pressures to 100 psig, using Swagelok compression fittings that make it transferable to the New Sampling/Sensor Initiative (NeSSI) platform. NeSSI is an industry initiative undertaken to standardize and simplify the design of systems used in chemical process analysis. Sensor system components are ATEX certified for operation in Class I/Div II Group B hazardous locations. The sensor's 4–20mA output can be readily integrated into existing data control systems. We expect that using this technology in the process industry would reduce the costs associated with the purchase, installation, maintenance, and replacement of hydrogen-specific sensors used in the industry's typical analyzer equipment (Figure 4).
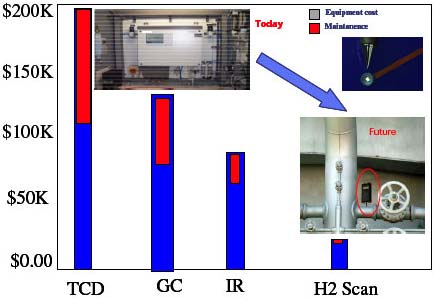 Figure 4. Comparison of costs for installation and maintenance for hydrogen process analyzers. TCD = thermal conductivity detector, GC = gas chromatography, and IR = infrared |
Sensor Applications
In certain industrial applications—such as hydrogenating cooking oil and hydro-treating petroleum crude into heating oil, gasoline, and diesel and jet fuels—it is critical to quantify the absolute concentration (partial pressure) of hydrogen in process operations to ensure process safety and operational efficiency. A typical refinery application for an inline hydrogen sensor is to measure the total hydrogen content in a mixed gas matrix (a mixture of hydrocarbons, CO2, H2, and other gases) as a function of time. The H2scan inline process hydrogen sensor allows direct point-of-use analysis of hydrogen concentration from 50%–100% v/v.
Hydrogen producers are also interested in accurate sensing as a key part of hydrogen management. For instance, within the pharmaceutical industry, multiphase hydrogenation reactions involving solid catalysts play a critical role. Accurate hydrogen monitoring is also needed in hydrotreating and in hydrogenation processes in hydrogen production facilities.
Hydrogen monitoring in nitrogen atmospheres can be useful for atmosphere control in semiconductor industries. A hydrogen-nitrogen protective atmosphere (rather than ammonia) in heat-treating operations provides a safer, nontoxic alternative to dissociated ammonia in metal- and materials-processing applications and provides improved reliability, better regulatory compliance, improved safety, lower maintenance requirements, and lower capital costs. Currently, hydrogen measurements are not taken at every needed monitoring point because established monitoring techniques (e.g., mass spectrometry and gas chromatography) are cost-prohibitive for multipoint installations. The H2Scan inline hydrogen sensor can enable these applications.
During molten aluminum processing, aluminum can react with moisture to form aluminum oxide and hydrogen. Hydrogen is far more soluble in liquid aluminum than it is in solid aluminum and can cause porosity during solidification if the concentration of dissolved hydrogen is too high. A proposed use of these new process hydrogen sensors is to quantify the hydrogen content directly from the nitrogen gas used to blanket the molten aluminum, which may allow the process control system to respond to the hot off-gas at an earlier stage during the process. In chlorine manufacturing, energy generation, nuclear power plants, and power transformers, hydrogen monitoring is important in preventing failures and explosions. Monitoring is also important in hydrogen refueling stations, service pump areas, and in the engine compartment of hydrogen-powered fuel cell vehicles.
Hydrogen has the potential to be an important source of clean fuel in our energy-driven economy and the use of accurate hydrogen monitoring is a key component of several of its emerging areas (Figure 5). Inline hydrogen-specific sensors offer an attractive replacement for traditional process analyzers, with reduced costs of ownership, installation, maintenance, and replacement. H2Scan's sensors, with their broad measurement range, hydrogen-specificity, and ability to operate with contaminant backgrounds, can enhance safety monitoring in the oil and gas, petrochemical, refinery, and nuclear facilities and aid the optimized production, storage, and delivery of hydrogen so important to the energy efficiency of these industries.
 Figure 5. Hydrogen-specific sensor process and safety applications in the emerging energy economy |