We all know that electronic devices get hot, and that spray cooling can calm them down. But just how does this technique work? Understanding the physics of spray cooling will be essential as work goes on to build ever-smaller electronic systems, circuits, and chips.
 Stephanie vL Henkel |
The problem with densely packed electronic devices such as chips and ICs is that the heat they generate has nowhere to go. As these devices shrink, so do their avenues of heat dissipation. A PC, for instance, produces <1 W/cm2 . A heat sink or fan can handle that, partly because the PC is not encapsulated. But when it comes to micro-devices that produce hundreds or thousands of watts per square centimeter, engineers rely on spray cooling, a high-flux, heat-removal technique that applies water to the individual chips.
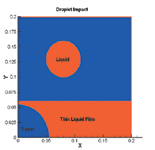 Droplet and liquid film at initial conditions |
At the University of Arkansas, researchers led by Panneer Selvam have created computational models of the effect of spray cooling on heated surfaces. Their conclusions indicate that the phenomenon is a result of a complex interaction of conduction and convection. Selvam also determined that the density of individual droplets affects the ability of each drop's ability to cool a heated surface.
Heat produced by a chip creates a thin, liquid film and vapor bubbles on its surface. The droplets in a fine mist of coolant spray interact with the film and the bubbles to transfer heat. The researchers created a computational model of a droplet's flow and interaction with the film or bubble that allowed them to study fluid properties, such as density, viscosity, and thermal conductivity. The complex equations they developed led to a better understanding of a droplet's effect on gravity, surface tension, and phase change.
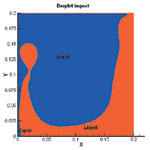 Liquid and vapor regions at 131.2 µs |
To study the impact of a droplet on a thin liquid film with a growing vapor bubble, initial conditions were established of a bubble radius of 40.491 μm in a liquid layer of 44.172 μm. Then, to explain the mechanism of heat removal, the configuration of liquid and vapor, temperature contour in the computational region, and variation of the Nusselt number on the heated surface were plotted for that configuration. It turns out that upon droplet impact the liquid layer above the bubble tears away and the bubble merges with the vapor above the liquid. So, when the bubble breaks, the cooler liquid spreads into the dry hot area and heat is conducted from the wall to the liquid layer.
Selvam also discovered that a droplet's density affects its ability to cool the surface. At low-density ratios, the droplet failed to burst vapor bubbles within the liquid film. At high-density ratios, droplets burst vapor bubbles upon impact and thus allowed the process of conduction and convection to begin. In other words, energy created by the interaction of the droplet with the thin liquid layer and vapor bubble caused heat to convey or transfer away from the surface.
Findings thus far have come from 2D models; future work will focus on 3D models of surface tension between liquid, vapor, gravity, and viscosity.
The work is supported by the Office of Naval Research, the U.S. Air Force, and NASA.
Contact Panneer Selvam, PhD, PE, Director, Computational Mechanics Lab, University of Arkansas, Fayetteville, AR; 479-575-5356, [email protected], www.uark.edu.