Analog Devices passed a milestone recently with FDA 510 (k) clearance and commercial launch of Sensinel, a remote, non-invasive Cardiopulmonary Management System.
The wearable is designed to help capture cardiopulmonary measurements for use in managing conditions such as heart failure without the need to implant a device under a patient’s skin. For ADI, it is the first FDA clearance the company has received in its nearly 60-year history.
For doctors and patients, the Sensinel CPM System holds promise as a way to reduce hospitalizations and treatments, now estimated to cost Americans $30 billion a year. The American Heart Association says 6 million Americans live with heart failure, a number expected to grow to 8 million by 2030.
Sensinel promises to help in early detection of physiological changes to help clinicians prevent a heart failure hospitalization, said Dr. Sean Pinney, chief cardiologist at Mount Sinai Morningside in New York City. He first began working with ADI on Sensinel five years ago and has recently been able to see how the device is accurate and reliable. The product is being evaluated at other hospitals as well.
ADI argues that other products on the market are not specific enough to provide data that clinicians need for early intervention.
Heart failure patients wear the Sensinel adhesive device on the chest for a few minutes daily to provide a set of readings, all of which can be transmitted to clinicians for analysis over time.
“Clinicians are able to detect psychological signals at an earlier time and that’s unique,” Dr. Pinney told Fierce Electronics. “Multiple signals are integrated into a score clinicians can use.” Early signals can show subtle indications of emerging problems.
The data generated by Sensinel can be shared with a team of clinicians for a needed collaborative approach, he said.
Based on his own experience, Dr. Pinney said the Sensinel offers multiple signals—“things that align everyday better than what I can do when listening to heart sounds…below the acoustic level of what I can hear.”
He added: “Heart rate variability, bioimpedance, how much congestion there is in the chest are all the things I can measure, but [Sensinel] can do it repeatedly and every day, reliably, so it’s an extension of my exam of a patient.”
For a patient with heart failure, a visit to an ER can cost $2,000. “If we can keep people out of the ER and keep them at home, that’s advantageous to patients for sure,” Dr. Pinney said. “Nobody wants to do an ER visit in New York City.”
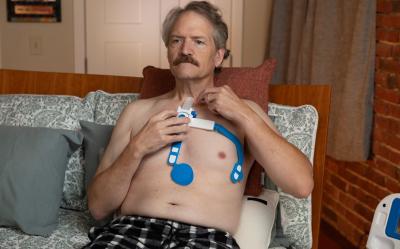
On the technical side, Sensinel is worn using disposable adhesives by a patient at home for three to five minutes a day, allowing multiple sensors to collect patient data. That data is uploaded to a base station in the home and then transmitted over cellular to ADI’s Sensinel CPM Cloud platform. A clinician can use a mobile app to collect a baseline reading during initial device deployment, with that data analyzed in the cloud using ADI algorithms.
The sensors on the wearable capture multiple heart failure- specific indicators such as: diastolic heart sound strength, heart sounds, heart rate, relative tidal volume, temperature, thoracic impedance, respiratory rate, body posture and single-lead ECG to measure heart, lung and other functions.
ADI said pricing is based on a subscription model, but did not offer details. ADI offers more information on its website:
RELATED: Wearables, wearable sensors gain popularity with pandemic, despite 2020 revenue lull