Ion mobility spectroscopy (IMS) is a leading technology for the on-site detection of trace quantities of chemicals—such as chemical warfare agents (CWAs), explosives, and narcotics—in air or water. In the 1980s, NASA chose IMS for air-quality monitoring aboard the international space station. Current Department of Defense (DoD) contracts in the U.S. and the U.K. and next-generation NASA air-quality monitoring systems are using differential mobility spectroscopy (DMS). DMS, although it is classified as an IMS-related technology, is quite different in its underlying physics and in its detection capabilities. This article will discuss the principal characteristics and operation of IMS and DMS, including some of the advantages of DMS.
Ionization Technology Sensors
Ionization is the process of converting an atom or molecule into its component ions; each chemical species produces a unique set of ions. Both IMS and DMS rely upon ionization chemistry to detect the presence of a given chemical species (Figure 1). Ionization sensor sources may be either radioactive—generally nickel (63Ni) or Americium (241Am)—or nonradioactive, e.g., UV, plasma, and now carbon nanotube technology. Once the sample is ionized, the objective of both the technologies is to separate and differentiate one ion species from another. After separation, the sensor detects and quantifies one species from another. Finally, the detection and quantification are presented as data.
Figure 1. The logic behind ionization mobility |
IMS Basis of Operation
IMS identifies and detects chemicals based on time-of-flight (TOF) principles (Figure 2). Essentially, IMS measures the time it takes a certain chemical ion to move through a uniform RF electric field. Samples are ionized and the sample's ions are lined up using a shutter mechanism and then floated into a drift tube. Because it lines up the ions, IMS uses only a very small percentage (~1%) of the sample.
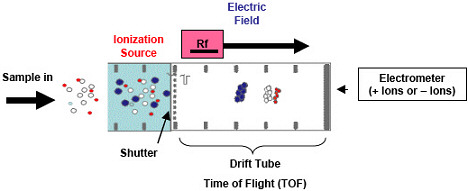 Figure 2. The principle of ion mobility spectroscopy (IMS) |
The drift tube contains a homogenous electric field and this field moves the ions down the drift tube. The homogenous electric field duty cycle can be set to more than hundreds of volts per centimeter, depending on the class of compounds targeted. Once set, the field does not change. The ions interact with neutral molecules within the drift tube, resulting in a TOF dependent on ion mass, size, and morphology. Once a target's TOF is established, the identification is based on that known TOF.
Early IMS systems generally operated in either a positive or negative ion mode. In contrast, recent IMS developments use oscillating negative and positive polarities in the drift tube to capture both negative and positive ion information through sequential operation. This means that a compound's negative and positive ions are determined in different sequential sample events. Quantification is based on the TOF response of an electrometer or Faraday plate.
The strength of IMS is its ability to quickly separate the ions of one chemical species from those of another, typically taking 3–15 ms per measurement. This attribute, coupled with relatively high sensitivity and relatively small size (4–5 in. long), has made IMS a natural fit for detection of explosives, narcotics, and chemical warfare agents, applications in which the overwhelming need was speed of analysis. When time is of the essence, one can't afford to wait 15 minutes for results from a gas chromatograph or deal with a high rate of false positives with less sensitive electrochemical cells. Because IMS uses TOF, increased selectivity requires a longer drift tube. However, longer drift tubes require more power and increase the form factor of resulting devices.
DMS Basis of Operation
DMS identifies and detects chemicals based on a chemical species' ion mobility in low and high electric fields (Figure 3). Samples are ionized and then flowed continuously via a carrier gas, such as air, into the detector area with its parallel plates spaced 0.5 mm apart. Once in the detector area, the ions experience a uniform oscillating asymmetric radio frequency electric field (Rf) which is typically 1 MHz and ranges from 500–1500 V. As applied, the Rf causes a perpendicular motion of the ions, resulting in a zigzag motion. Each ion species will exhibit discrete mobility characteristics.
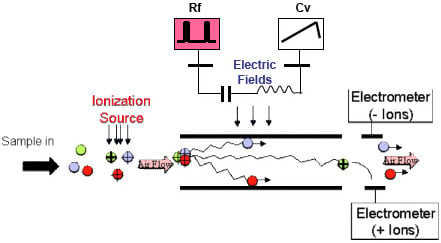 Figure 3. The principle of differential ion mobility spectroscopy (DMS) |
To make the DMS sensor tunable, a perpendicular DC tuning field, known as the compensation voltage (Cv), is applied. This field is superimposed on the oscillating asymmetrical Rf field and keeps the ions of interest centered between the parallel plates and is detectable simultaneously by both negative and positive electrometers. The electric field conditions required to permit a particular ion to pass though the filter to the detector are specific to each ion species.
The DMS device can be operated in several modes. When functioning as a programmable chemical filter, the compensation voltage is fixed such that only one particular ion species is permitted to reach the detector. The charges received at the detector can be integrated for a selected period of time, improving the SNR and enabling significantly higher sensitivities. Alternatively, when operating in spectrometer mode, the compensation voltage can be scanned across a number of compensation voltages to allow various ions of interest to pass to the detectors.
The resident time for ions in the ion filter region is typically 1–2 ms with a transport gas flow rate of 300 ml/min. This rapid flow rate, coupled with the heating of the sensor (80°C–120°C), minimizes the risk of build-up of any material between the plates.
Key Benefits
IMS still has important applications, including threat detection, first responder use, and explosive detection, but DMS offers several advantages, including:
- Greater sensitivity through continuous sampling. IMS uses a shutter to enable timing of motion, which results in 99% of the sample being discarded and, thus, lower sensitivity. Typically, DMS is about 10–100 times more sensitive than IMS, detecting in the parts-per-billion to parts-per-trillion range.
- More data for enhanced chemical identification. DMS can simultaneously detect both positive and negative ions whereas IMS can only detect one or the other. When combined with a range of Rf and Cv combinations that can be switched very rapidly, DMS results in a more data-rich environment and eliminates overlapping peaks, reducing both false positives and false negatives.
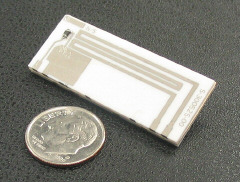
Figure 4. The Sionex differential mobility spectrometer detector - Smaller size. With an overall sensor length of about 1 in. (Figure 4), DMS is physically smaller. Since IMS relies on differences in TOF for its operation, reducing the size of the drift tube reduces its resolution.
- Lower cost. DMS is microfabricated using methods that are capable of mass production. In contrast, IMS fabrication often involves significant manual assembly, adding to the device's cost and complexity.
Originally, IMS was the technology of choice for threat detection because of its ability to quickly separate and reliably detect target chemicals. IMS technology matured as new threat detection needs surfaced, and was enhanced to provide sequential detection in both negative and positive channels to detect both negative and positive ions. However, it could not keep up with ever-expanding lists of explosives and chemical warfare agents (CWAs). When Alliance forces entered Iraq in the Gulf War in 1990 and in the Iraq war in 2003, it became clear that detecting CWAs in a war environment was not that easy. The IMS systems used had unacceptably high false-positive rates, especially in the presence of organophosphate pesticides, and suffered interference when exposed to burning diesel fuels and AFFF (aqueous firefighters foam). Further, when "shoe bomber" Richard Reid was caught on an airplane trying light a fuse to a shoe that hid pentaerythritol tetranitrate (PETN) plastic explosives, the authorities and the public quickly learned that then-current IMS detectors could not detect triacetone triperoxide (TATP) detonator at effective levels of sensitivity. The use of TATP as a detonator confirmed to intelligence personnel that terrorist elements knew the limitations of current explosive-detection technologies.
IMS offers reasonable sensitivity and selectivity at very quick speeds—something the other detection technologies do not provide. However, by the middle of this decade, it was clear that the technology needed an overhaul. The government led the way with programs to find a replacement for IMS or enhanced technology that had the speed of IMS but with higher sensitivity and smaller size. Governments and program managers running CWA programs learned of DMS, which offers the speed of IMS but with greater selectivity and lower sensitivity, and had sensors initially about a quarter the size of current IMS systems. This article has explained the basic physics of IMS and DMS systems. Next month we will discuss how DMS and IMS, used simultaneously, can offer greater speed, selectivity, and sensitivity.