In January 2017, the Maryland-based BrainScope Company Inc. released its Ahead 300 product. Approved by the US Food and Drug Administration (FDA) in September 2016, Ahead 300 is used to provide objective assessment of mildly presented head injuries across a full spectrum of brain injuries for up to three days following injury. Essentially, it’s intended to help clinicians determine the proper disposition of the patient by directly addressing two key questions in making a diagnosis:
- Is it likely that the mildly presenting head-injured patient has a traumatic structural brain injury, which would be visible on a CT scan?
- Is there evidence of something functionally abnormal with the brain after head injury, which may be concussion?
To answer these two key questions, the Ahead 300 leverages handheld technology and a proprietary disposable sensor headset. The Ahead 300 features BrainScope’s patent-protected electroencephalography (EEG) capabilities utilizing advanced signal processing, sophisticated algorithms and machine learning to help in the evaluation of head-injured patients with objective biomarkers of brain electrical activity.
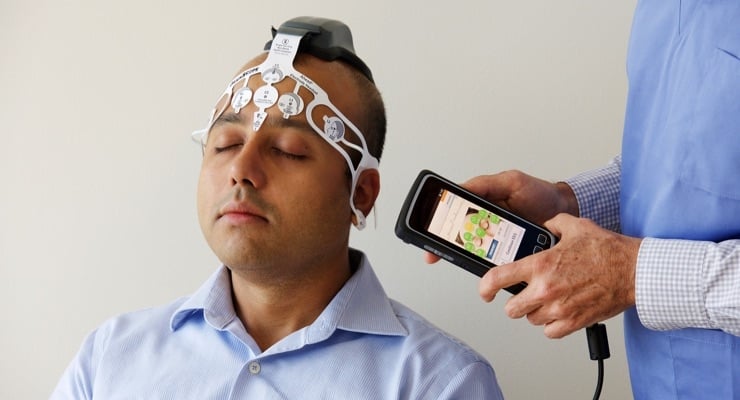
Ahead 300 leverages handheld technology and a proprietary disposable sensor headset.
Interestingly, Ahead 300 was developed in partnership with the US Department of Defense through six research contracts, and with more than 20 clinical studies at 55 sites and 16 peer-reviewed publications.
Attesting to the system’s viability, a recently released peer-reviewed publication demonstrates that Ahead 300 significantly improves prediction of traumatic brain injury (TBI) beyond standard clinical risk factors currently in use. The publication demonstrates that prediction of a CT positive TBI is significantly improved when the electroencephalography (EEG) based biomarker from Ahead 300 is used in addition to the most commonly considered predictive risk factor: loss of consciousness (LOC).
The results of this study entitled “Increased prognostic accuracy of TBI when a brain electrical activity biomarker is added to Loss of Consciousness (LOC),” were published in the peer-reviewed American Journal of Emergency Medicine. Results of this study show an 83% improvement in the prediction of TBI using the Ahead 300 compared to that obtained using a history of LOC alone or LOC plus traumatic amnesia, the two most commonly used clinical diagnostic indicators for TBI.
For more information, visit BRAINSCOPE.