
Carbon electrodes are extensively used in many electrochemical sensors, with the carbon primarily sp² in nature. Of increasing interest are the potential applications for sp³ carbon i.e. diamond in electrochemical sensing.
In its synthetic form, diamond is predominately used as an abrasive in industrial applications. One of its lesser known properties is that it can be doped with boron to provide semiconducting or even metal-like conductivity characteristics, dependent on the dopant level.
Boron doping turns the diamond from transparent to blue (semi-conducting) to black (metal-like). When combined with its inert surface characteristics, boron doped diamond (BDD) electrodes have intriguing electrochemical properties. These include the widest solvent window of all electrode materials in aqueous solutions, low background and capacitive currents, reduced fouling, and the ability to withstand extreme potentials, all while retaining the excellent mechanical robustness diamond is famous for. Thus BDD is slowly rising in prominence in the electrochemical arena, predominantly where longevity and low maintenance are key attributes.
BDD is typically produced using a type of chemical vapor deposition (CVD) process, where polycrystalline wafers, typically 100 mm to 130 mm in diameter, of BDD are grown, outside the normal high temperature and pressure region of the phase diagram for diamond (see reference 1). When manufactured to be thick enough to be free-standing, typically >0.4 mm for a 130 mm wafer, free-standing BDD makes a very robust electrode (see figure 1).

Fig. 1
One of the simplest exploitations of BDD's remarkable physical and electrochemical properties is to use the electrode under extremely high oxidation potentials for the generation of hydroxyl radicals in advanced oxidation processes, used for water treatment applications (see reference 2). Typical electrodes materials other than BDD would simply corrode away.
Diamond Sensor Devices
BDD is not a typical electrode material and research by material scientists, chemists. and physicists has been required to understand, optimize, and truly harness these exceptional properties in sensing applications (see references 3 and 4). With precise control of the phase purity and boron doping level, combined with surface processing for a precision polished surface, BDD electrodes can exhibit high quality electrochemical properties. These include a solvent window >3.5 V, a capacitance of <10 µF cm-², and fully reversible, <70 mV, electron transfer characteristics with classical outer sphere redox couples (see figure 2).
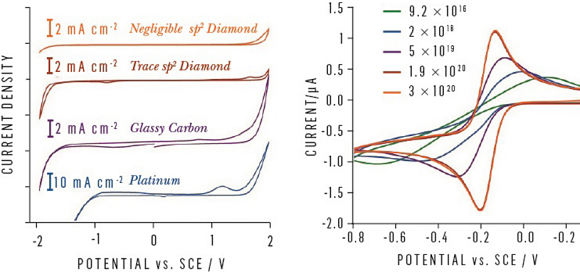
Fig. 2
These attributes make conducting diamond ideal for many electrochemical based sensing applications (see reference 5)
Currently
Very recent developments have resulted in a new manufacturing process where multiple BDD electrodes of any geometry can be integrated into insulating diamond substrates to produce individually addressable all-diamond sensor devices (see figure 3a). The growth and packaging procedure dictates that the BDD electrodes are free from catalytic sp2 carbon, which can result in reduced solvent windows and higher capacitive currents.
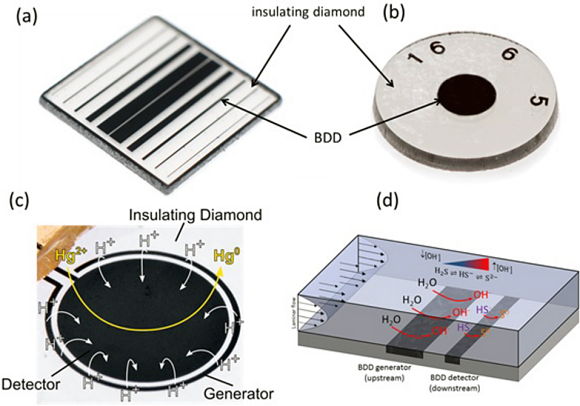
Fig. 3
These sensors can be used for many different and direct (electrochemical) sensing measurements without the need for membranes (which are prone to clogging) and filters (see reference 6). The all diamond electrodes can function in even the harshest of environments, offering detection sensitivities at the ppb level (see reference 7).
New Sensing Methodologies for In-situ Measurements
The robustness of BDD and stability of the wide solvent window means that extreme potentials can be applied to these devices to enable self-cleaning of the electrode surface and, if required, control of the local pH of the measurement environment (see figure 3b). The latter enables optimization of the electrochemical signature and does away with the need for buffered solutions used in conjunction with membrane electrodes, or independent measurement of solution pH.
This excitingly paves the way for use of these sensors in in-situ environments with long term placement at the source a real possibility. Figure 3a and c shows two diamond sensors; a multiple band electrode sensor, which also functions as a conductivity sensor and a ring – disc sensor (see reference 6).
For both the black tracks are BDD and the transparent areas, insulating diamond. A dual electrode sensor has been used, for example, as a means of controlling the local pH environment of the sensing disc electrode. The outer, or upstream, BDD electrode (in a fluidic flow cell), can be used to electrochemically break down water, creating a controlled pH environment over the detector electrode. These structures have been successfully deployed to detect heavy metals (see reference 7) and dissolved hydrogen sulfide (see reference 8) in water in far from ideal pH conditions.
New Electrochemical Sensing Technology
Although BDD electrode materials have been available for some years, this is the first time that diamond materials have been fully optimized for electrochemical sensing applications and robustly packaged to survive in real environments. As a first step in the development of these BDD electrochemical sensors, Element Six has developed a co-planar all diamond (BDD insulated in insulating diamond) macro-electrode (see figure 3b). It is designed for electrochemical research applications that can be treated and used in the same way as conventional electrodes, such as platinum, gold and glassy carbon.
These materials and devices will enable new applications for electrochemical sensors that are directly in the environment of interest, particularly in harsh and permanent applications. Even in established electrochemical measurements, the exceptional properties of BDD all diamond sensors will push performance to much higher levels.
Multiple electrode devices enable new sensing applications and hold the prospect for fully integrated sensor devices that can manipulate the local environment and perform several measurements in one device. However, full exploitation of these unique BDD sensor devices will require a partnership with aqueous analytical instrumentation companies to develop and leverage the technology and IP that has been generated.
References
1. R S Balmer et al J. Phys.: Condens. Matter 2009 21 364221
2. Advanced oxidation processes for water treatment: advances and trends for R&D 2008 Comninellis C et al, J. Chem. Technol. Biotechnol. 2008 83: 769–776.
3. L. A. Hutton, J. G. Iacobini, E. Bitziou, R. B. Channon, M. E. Newton, J. V. Macpherson, Anal. Chem. 2013, 85, 7230-7240
4. H. V. Patten, L. A. Hutton, K. E. Meadows, J. G. Iacobini, D. Battistel, K. McKelvey, A. W. Colburn, M. E. Newton, J. V. Macpherson, P. R. Unwin, Angew Chemie Intl. Ed. 2012, 51, 7002-7006
5. J.V. Macpherson, Phys.Chem.Chem.Phys. 2015, 17, 2935-2949
6. M. B. Joseph, E. Bitziou, T. L. Read, L. Meng, N. L. Palmer, T. P. Mollart. M. E. Newton and J. V. Macpherson, Anal. Chem. 2014, 86, 5238-5244
7. T. L. Read, E. Bitziou, M. B. Joseph and J. V. Macpherson, Anal. Chem. 2014, 86, 367-371
8. E. Bitziou, M. B. Joseph, T. L. Read, N. Palmer, T. Mollart, M. E. Newton and J. V. Macpherson, Anal. Chem. 2014, 86, 10834-10840
About the Authors
Julie Macpherson is a professor Department of Chemistry, University of Warwick and holds and Royal Society Industrial Fellowship in partnership with Element Six. Her research focuses on the development of sensors based on different forms of carbon, including conducting diamond, carbon nanotubes and graphene, with a range of applications in environmental monitoring, healthcare technologies and water research. She has published over 150 papers and 14 patents.
Tim Mollart is a Product Marketing Manager at Element Six Ltd. He has a background in research and development, specializing in the field of diamond optics and diamond electrochemistry and is the industrial sponsor of Professor Macpherson's Royal Society Fellowship He has published over 30 paper and patents.
Related Stories
Capacitive Pressure Sensors for Medical Applications
Silicon Carbide Microsensors for Demanding Applications