What has a surface area the size of a football field, structural features smaller than a pinpoint, and can be custom designed to detect specific chemicals for integration into a superior sensor? If you guessed functional multiscale materials—also known as nanoscale materials—you would be correct. What are these amazing materials and how will they revolutionize sensing technology?
Because of the minuscule size of nanoscale materials (1 nm = 10–9 m), their chemical and physical properties differ from those of their bulk counterparts and therefore behave differently. One of these properties is an ability to be "functionalized" or custom-designed to attract specific molecules; another is an extremely high surface area tucked into a tiny space.
The unique characteristics of nanoscale materials make them a perfect fit for the sensor world. Integrating them into existing sensors can increase the devices' sensitivity, selectivity, and speed. In addition, the large surface area and low volume greatly facilitate sensor miniaturization.
 |
Researchers at the Department of Energy's Pacific Northwest National Laboratory (PNNL) are integrating functionalized nanoporous silica and carbon nano-tubes—both nanoscale materials—into a variety of sensor applications to meet urgent needs in fields ranging from biomedicine and environmental remediation to national security. The scientists' goal is to lay the foundation for a miniaturized sensor that uses the smallest sample to detect the smallest concentration of molecules of interest. "Ideally we'd like to be able to just walk into a room and find one molecule of what we're looking for with a sensor the size of a pinhead and say, 'it's here' without concentrating or distilling all of the air in the room," says Tim Bays, a PNNL scientist who works on the functional multiscale materials team.
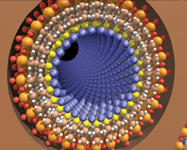 Figure 1. Self-assembled monolayers on mesoporous supports (SAMMS), based on a coating process that makes silica bind to selected metals; among potential applications: remediation, water treatment, catalysts, and controlled-release markets |
While researchers admit that they have a long way to go before they can effectively achieve this goal, they have made progress in the laboratory. According to Shane Addleman, another member of the team, "We have demonstrated a million-fold improvement in sensors for detection of metals, radionuclides, and gases with the correct integration of nanoscale materials."
Nanoporous Silicas—Selective, Fast, High-Loading
One particularly promising area of nano R&D is a functionalized nanoporous silica called self-assembled monolayers on mesoporous supports (SAMMS, Figure 1), the work of PNNL's Glen Fryxell and Jun Liu. Nanoporous silicas, such as the ones used as the scaffolding in SAMMS technology, contain millions of tiny pores. The nooks and crannies of the pores provide extremely high surface area in a low volume—one tablespoon of SAMMS contains the surface area of a football field, ~5000 sq. yd., which can be loaded with reactive sites where molecules can bind. "The beauty of functional multiscale materials is the ability to make all that space usable," Bays says. And that is done by "functionalizing" or designing a monolayer that is attached to the pores and that seeks out certain substances.
 Figure 2. Small amounts of thiol-SAMMS can adsorb an extremely large quantity of mercury, which then becomes inert |
Thiol-modified SAMMS has been designed to capture mercury and has also shown an affinity for binding with other "soft" heavy metals, including lead, cadmium (a toxic component of Ni-Cd batteries), gold, silver, and copper. Other types of SAMMS have been designed to capture chemicals such as arsenic (arsenate), chromium (chromate), and radionuclides. The bottom line is that specific functional groups can be designed to target specific ions or molecules. Yuehe Lin is leading a PNNL project for the DOE Office of Science's Environmental Management Science Program to develop SAMMS-based electrochemical sensors for detecting and quantifying toxic metal ions, including mercury, cadmium, copper, lead, and uranium in mixed waste and groundwater.
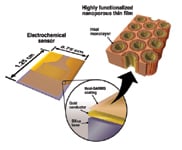 Figure 3. Thin-film SAMMS can be used as a coating on the electrode surface of electrochemical sensors |
One of the greatest benefits of functionalized SAMMS is its ability to preconcentrate. "The concentration of some metal ions in nature may be as low as two or three parts per billion—too low to be detected without preconcentration," says Wassana Yantasee, a PNNL engineer working on this project. Because SAMMS can be designed to "cherry pick" specific molecules out of solution and retain them in its pores, it can effectively preconcentrate even quantities down to 0.1 ppb, according to Bays. As Yantasee explains, "SAMMS used as an electrode coating preconcentrates the substance we want to detect right on the electrode surface, which is an important component of electrochemical sensors." Preconcentration also allows sensor portability. "Most in situ detection methods currently used do not preconcentrate," Bays says. "Typically, you need a lab to preconcentrate and that involves going out, scooping up a liter of river water, transporting it, and then concentrating it at the lab."
Although the up-front costs may be higher in some applications, SAMMS's ability to be specific can offer considerable savings in environmental applications (Figure 2). PNNL scientists put the costs at one-fourth that of using a commercial resin to dispose of mercury and an even better ROI over activated carbon. The smaller amount of SAMMS material required to capture the mercury reduces the cost of disposal.
SAMMS-Based Sensor Platforms
Yantasee and Lin are working on three sensor platforms. Thin-film SAMMS (Figure 3) is a coating that can be applied to the electrode surface of a sensor. It works on any type of surface, and PNNL researchers are using it on microchip electrodes. This could be a first step toward miniaturizing sensing devices.
A second platform involves disposable screen-printed electrodes that have been coated with SAMMS mixed with a conductive material. These electrodes are convenient and inexpensive; they can be dipped into a solution for a quick reading on the concentration of certain metal ions and then discarded. Their potential applications include diagnostics by health professionals in countries where medical facilities are not readily available and in detecting biomarkers for diseases.
Yantasee and Lin are also developing an automated, portable sensor unit. At present it is about the size of a shoebox and incorporates a microchannel device in which samples and reagents are programmed to flow in sequence over the electrode surface for a specified period of time, ~5 s, before providing a reading. The sensor can be set to take readings at specified times during the day.
The next step will be a remotely controlled unit for use in nuclear proliferation activities to detect radioactive substances where there is risk of human exposure.
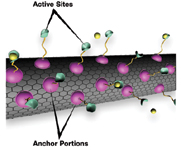 Figure 4. A new way to attach molecules to the surfaces of carbon nanotubes entails a "supercritical fluid" (not shown) with both gas- and liquid-like properties to load specially designed "anchor molecules" onto the nanotubes without compromising tube strength and sensitivity; the active sites are indicated here binding to a targeted chemical (yellow) |
Fine-Tuning Carbon Nanotubes
SAMMS material's behavior is a function of its extensive interior surface area, while that of carbon nanotubes—fine carbon filaments—is based on their exterior surface (Figure 4). "You can put a lot of them together so you get the same benefits of low volume and high reactivity of SAMMS," Bays says. "Their strong suit is specificity and electrical conductivity (Figure 5). Because carbon nanotubes are conductive, they can provide a signal each time a target substance binds to the enzyme attached to the nanotube."
PNNL researchers are putting nano-tubes to work as biosensors and improving the way they can be chemically customized to form the basis for a wide variety of devices, including atmospheric and blood sensors. Yuehe Lin and Guodong Liu fashioned carbon nanotubes into a portable, automated sensor system for organophosphate (OP) detection. Besides posing a serious environmental hazard, OP compounds are the raw materials for nerve agents. Detecting these compounds could give emergency personnel a head start in responding to a terrorist attack in which such agents are used.
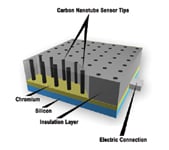 Figure 5. Carbon nanotubes offer specificity and electrical conductivity |
To make these extremely sensitive sensor materials, Lin and Liu ued a layer-by-layer self-assembly technique to attach enzymes to the surface of carbon nanotubes. These are the same enzymes present in neurotransmitters, the impulses that enable nerve cells to communicate. Next, they prepared a 3 mm dia. sensor with the carbon nanotubes and their bound enzymes. Enzymatic activity is damped in the presence of OP. Acting as electrodes, the nanotubes detect the inhibition as a muted signal and pass that information to an off-the-shelf electrochemical detector. The detector was plugged into a notebook computer for an instant reading of OP at concentrations down to 1 ppt. Most existing OP sensors respond to levels between parts per million and parts per billion. (A paper based on the results has been accepted for publication in Analytical Chemistry.)
Testing another sensor configuration, Lin, colleagues from PNNL, and researchers from Boston College demonstrated that carbon nanotubes could be used in broad biomedical applications such as measuring blood sugar. In a paper published in the February 2004 issue of Nano Letters, Lin described how he stood carbon nanotubes ~50 nm in dia. on end (Figure 6). They were treated with the enzyme glucose oxidase and anchored to an epoxy-covered material that acted as an electrode contact. The tips of the enzyme-covered nanotubes protruded through an insulation layer, allowing them to come into contact with blood. Sugar in the blood started a catalytic reaction, the energy from which was conveyed via the carbon-nanotube electrodes. The stronger the signal, the higher the blood sugar level.
Lin credits the sensor's utility to the reactivity of the enzyme, the excellent conductivity of carbon nanotubes, and the enormous nanoelectrode array—about 1 million nanotubes integrated on a 5 3 5 mm microchip electrode. The next step will be to integrate the microchip electrode into a portable unit to work with blood samples.
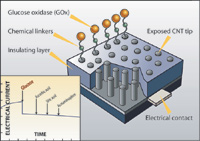 Figure 6. To create a blood glucose sensor, carbon nanotubes were treated with glucose oxidase and anchored to an epoxy-covered material that acted as an electrode contact |
Vision
With their ability to react rapidly and with extreme sensitivity, functional multiscale materials may dramatically improve sensing technology. These advances and others possible with engineered applications of functional multiscale materials will open many new opportunities for sensors in environmental, biomedical, national security, and industrial arenas.
Virginia Sliman can be reached at Pacific Northwest National Laboratory, Richland, WA; 509-375-4372, [email protected], www.pnl.gov
More About SAMMS
Self-assembled monolayers on mesoporous supports (SAMMS, Figure 7) is a combination of two novel technologies: a nanoporous ceramic substrate (left), with a regular hexagonal array of nanopores (~6.0 nm), and a contaminant-selective self-assembled monolayer (right) with its cross-linked silane end attached to the pore surface and the functional moiety constituting the top surface of the monolayer.
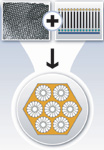 Figure 7. SAMMS is a combination of two novel technologies whose components are shown here |
The resulting material has all the pore surfaces lined with a functional monolayer (bottom) consisting of single layers of densely packed molecules custom designed to detect mercury, lead, chromium, and other toxic or precious metals. SAMMS can be tailored chemically to selectively bind to a wide range of contaminant types, based on the type of monolayer applied. It can be used effectively in water and non-water (hydrocarbon) solution streams.
Virginia Sliman can be reached at Pacific Northwest National Laboratory, Richland, WA; 509-375-4372, [email protected]