Even devotees of classical science fiction, with its blue-sky descriptions of marvels to come, must be awed by current research into devices that will allow quadriplegics to manipulate objects with signals from their brains. One of these, BrainGate, has been successfully demonstrated on two patients. The trial is being conducted under an Investigational Device Exemption from the FDA, which provides for implanting the BrainGate for a period of 12 months into five patients with quadriplegia due to spinal cord injury, stroke, or muscular dystrophy. The year-long study for the first patient will end during June of this year. So far, this participant continues to be able to use thoughts to control a computer, and no adverse events have been observed.
 BrainGate neural interface allows quadriplegics to control computer cursors with their thoughts. |
The BrainGate neural interface system is a proprietary, experimental brain-computer interface consisting of an implanted sensor that detects brain cell activity, along with external processors that convert brain signals into a computer-mediated output under the subject's own control. The chip-sized sensor is implanted on the surface of the primary motor cortex, the area of the brain that governs voluntary motion. A small wire connects the sensor to a pedestal that extends through the scalp. An external cable connects the pedestal to a cart containing computers, signal processors, and monitors that allow the study operators to determine how well the participant can control his or her neural output.
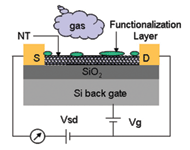 Here's the technology underlying the disposable CO2 sensor. |
The ongoing feasibility study will enroll up to five quadriplegic individuals between the ages of 18 and 60 who meet certain selection criteria. The two primary goals of the pilot clinical study are to characterize the safety profile of the device and to evaluate the quality, type, and usefulness of neural output control that patients can achieve using thoughts. After implantantion of the sensor, particpants will perform tasks such as attempting to move a computer cursor toward a specific target. After the study period is over, each participant can either have the device surgically removed or opt to participate in future studies.
BrainGate clinical sites include Sargent Rehabilitation Center (Warwick, RI), Spaulding Rehabilitation Center (Boston, MA), and the Rehabilitation Institute of Chicago (Chicago, IL). Although they are actively recruiting additional trial subjects, there is no plan to expand the number of sites for the initial trial. For specific information on BrainGate, write to [email protected].
 Short Takes |
Contact Jessica Duda, Cyberkinetics Neurotechnology Systems, Inc., Foxboro, MA; 508-549-9981, x-112,