Building on earlier work performed at Sandia National Laboratory (Sensors, January 2005, "How's Your Battery Holding Up?"), this article discusses how the fiber-optic sensor described can be used to measure the state of charge in lead acid batteries. We will review the basic principles of operation, discuss the development of this technology into a realizable device, and show results from the testing of two production batteries.
A State of Charge Sensor
The state of charge (SOC) sensor is a device for measuring the actual energy content or charge level in a lead acid or other liquid electrolyte battery. These batteries, which comprise the bulk of the storage batteries currently in use worldwide, require the plates of the battery to be immersed in a liquid or gel sulfuric acid solution. Batteries are made up of several interconnecting cells and the number of connected cells determines the battery's operating voltage. The conventional and most accurate method of determining the SOC in wet lead acid batteries uses a hydrometer to measure the specific gravity of the electrolyte. However, this requires fluid extraction from each cell of the battery—a messy, slow, and sometimes dangerous procedure. The SOC is determined by comparing the specific gravity measurements to reference levels for that particular type of battery.
Newer digital hydrometers have simplified the procedure somewhat, but they still require fluid extraction, and because this is a relatively slow process, on a practical level it can only be employed periodically. Other methods of SOC measurement rely on battery voltage, charge integration, or cell impedence with various degrees of accuracy, price, and ease of use. The sensor described in this article works by directly measuring the index of refraction of the liquid or gel in each cell via a probe that is directly and semi-permanently inserted into the battery cells to provide a continuous readout of SOC.
Principle of Operation
Light propagates through an optical fiber—with its glass or plastic core and a thin layer of cladding—and the efficiency of the propagation depends on the relationship between the index of refraction of the core and the cladding, respectively. Given that n1 is the index of the core and n2 the index of the cladding then, for efficient propagation, n1 > n2. As n2 approaches the value of n1 the optical wave suffers progressively greater losses.
The sensor's operation is analogous to that of a conventional optical fiber with two exceptions: the cladding of the fiber is the liquid under measurement and the core of the fiber may be a liquid in a tube or a solid uncladded optical fiber with an index of refraction that is appropriate to the liquid being measured.
In a lead acid battery, the SOC or energy content is linearly related to the concentration of the electrolyte, which is a sulfuric acid solution (or gel) in conventional wet batteries. As the battery is charged or discharged, the concentration of acid increases or decreases ranging from 40% to 10% by weight, depending on the type of battery. In most solutions, the index of refraction of the sulfuric acid solution is very nearly linear with respect to concentration. Therefore the battery's charge level is proportional to the acid concentration and this can be directly derived from the relative change in the index of refraction of the battery electrolyte. The battery's ability to actually deliver that charge or energy level to the load comes under the heading of battery state-of-health—a topic related to overall battery diagnostics not addressed in this article.
Putting the Sensor Through its Paces
In practice, the sensor is composed of a thin quartz tube filled with liquid and with a reflecting mirror at one end. An optical wave introduced into the core of the sensor propagates down through the liquid, is reflected by the end mirror, and propagates back up the core, where it is captured by a bundle of conventional optical fibers coupled to a photodetector. The losses induced in the optical wave depend primarily on the difference between the indices of refraction of the core liquid and surrounding electrolyte, although the material and thickness of the tube do play a minor role in the propagation characteristics. The relative change in index of refraction of the surrounding liquid is determined by noting the relative change in the detected signal level compared to known calibration solutions.
A practical incarnation of the device resembles a conventional battery cap. The configuration shown in Figure 1 is intended for a retrofit application into existing batteries.
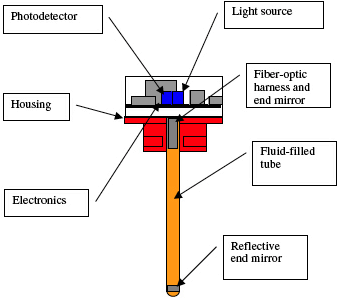 Figure 1. Notional sensor configuration in the form of a conventional battery cap |
The engineering prototype sensor shown in Figure 2 was built in this configuration and applied to two test batteries, an Exide E3600 and a Surrette S530. The SOC levels for a specific battery are typically referenced to specific gravity measurements and are supplied by the manufacturer. The Exide E3600 was subjected to a SOC measurement while subjecting the battery to a constant 15 A load current and periodically measuring the specific gravity with a digital hydrometer.
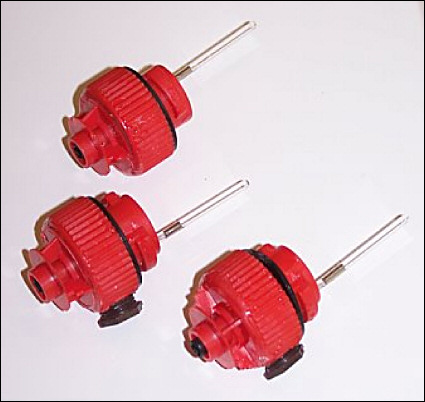 Figure 2. SOC sensor prototype configuration |
Although the manufacturer's specific gravity specifications were not available, the measurement results shown in Figure 3 were compared to the charge curve of a typical traction battery, which is used for motive power applications such as fork lifts and golf carts. We used the minimum and maximum solution concentration appropriate to the corresponding specific gravity values to calibrate the sensor. From our results, the sensor demonstrates good linearity and accuracy through the range of values from minimum to maximum charge.
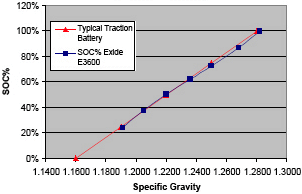 Figure 3. SOC test results on the Exide E3600 |
Next, we tested a 6.3 V Surrette S530 battery using simultaneous measurements of the battery's three cells (Figure 4).
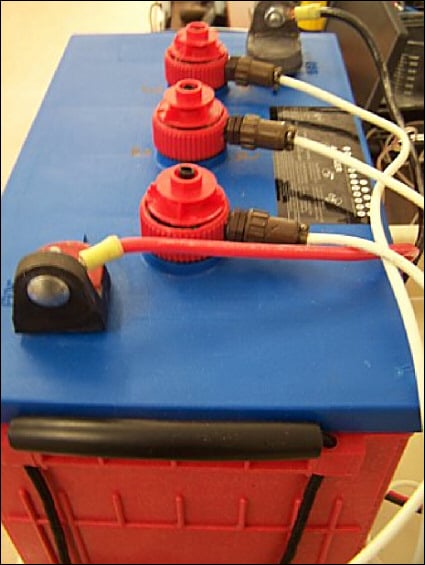 Figure 4. Surrette S530 battery equipped with prototype SOC sensors |
In this case, we had the manufacturer's specifications for specific gravity vs. SOC and again we calibrated the sensor using corresponding concentrations of reference solutions. The results are shown in Figure 5. All three cells of the battery as measured by the SOC sensor tracked the "typical" characteristic curve for this type of battery rather well. The departure of cell 2 (which departed from the typical by about 10% at the high SOC levels) was subsequently determined to be a calibration error.
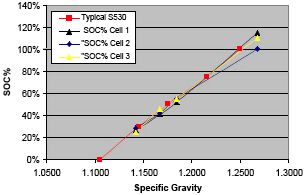 Figure 5. SOC test results on the Surrette S530 |
Closing Thoughts
In summary, the optical SOC sensing technology described herein appears to provide a new paradigm in charge sensing for a considerable proportion of the several hundred million lead acid batteries currently in existence. By eliminating the need to extract fluid from the battery cell(s), the design provides a rapid, real-time capability for SOC determination. Although the configuration tested was designed for retrofit applications in conventional wet batteries, the sensor can be incorporated directly into the battery at manufacture with corresponding applicability to sealed low-maintenance batteries.
ABOUT THE AUTHOR
Joseph S. Accetta, PhD, can be reached at JSA Photonics Inc., Corrales, NM; 505-897-3980, [email protected] or at Georgia Tech Research Institute, Albuquerque Field Office, Albuquerque, NM;; 505-897-3980, [email protected].
