
Introduction
Biomedical sensors exist at the interface between medical physiology and electronic engineering. To be clinically valid and useful, they must detect relevant biological phenomena and portray the data produced in a manner intuitively understandable and actionable by medical practitioners. To be technologically achievable and reliable, they must be designed and built using the best engineering methods, components, and manufacturing techniques.
The catch is: who is prepared to do the discovery and invention needed to produce them? How does one become educated and skilled in the wide range of processes that must be combined in biomedical sensor systems? Are there any phenomena still needing to be discovered? Are there important new medical sensors still needing to be invented?
This is the first of a two-part series of articles on discovery and development of novel medical sensors. This first article describes our discovery and initial exploration of a new biometric signal obtained by detecting variations in absorption of two wavelengths of LED light in skin tissue. The second article presents some lessons learned about the process of preparing to discover and invent in this area of technology.
A New Biometric Sensor
This journal is directed toward engineers, and primarily focuses on sensors from the technical perspective. However, unless actively informed by clinicians, engineers are likely to be unaware of unmet medical technology needs. On the other hand, most clinicians are uninformed about possible technological solutions, and are trained to do the best they can with whatever is provided for them. Unfortunately, critical care of premature newborn infants is doubly compromised because of its very small economic market size and, therefore, very limited resources for medical technology development, and an understandably conservative leadership of subspecialist clinicians who are reluctant to suggest development of new technology approaches for this high-risk area of medicine.
During undergraduate education in microbiology, followed by medical school, pediatric residency, and entry into general pediatric physician practice, the author became increasingly aware of the growing gap between the capability of medical technology developed for adult patients, and the limited capabilities of the technology being offered for critical care of infants and children. Beyond the more obvious issues of adapting to the tiny physical scale, there are several critical health problems that are unique to premature newborn infants, which cannot be adequately prevented, detected, or treated with adapted adult-oriented technology. One of the most important of these areas involves the management of the use of oxygen.
The common assumption is that newborn infants are especially vulnerable to insufficient oxygen, or hypoxia, and that hypoxia directly causes brain damage, blindness, and intestinal injury. Unfortunately, as will be discussed below, we have discovered new evidence that these devastating injuries are more likely the result of delivering too much oxygen to vulnerable tissues under crisis conditions. This problem is turning out to be not so much an engineering challenge, as it is a lapse in inter-professional communication, and shows an urgent need for targeted discovery and invention of new technology.
Unfortunately, active R&D of pulse oximeter (SpO2) sensors had already transformed into manufacturing quality control and cost containment about the time clinical use with infants was started. Further innovation, specifically into reflectance mode sensors that would be more suitable for premature infants, was discontinued by Nellcor in 1989. The only available option for infant intensive care was, and continues to be, adult fingertip-style sensors placed on the foot or hand of the infant. This approach leaves the following uniquely-preemie medical needs unmet:
- Sensor motion artifacts result in excessive false alarms, and delayed and missed real alarms;
- No simultaneous and continuous means of comparing pre-ductal arterial blood oxygen saturation with post-ductal arterial blood oxygen saturation;
- No means of detecting tissue hyperoxia; and, therefore
- No rational means of preventing oxidative stress-related eye, brain, and gut injuries in preemies.
With little medical device industry effort in the development of oximeter sensors specifically to meet these unique needs of premature infants in 1989, the author began his quest for a suitable reflectance mode SpO2 sensor design. Placement of the sensor on the infant’s foot or hand is problematic, since these are very active sites, especially during crying episodes when the oxygen status of the infant may be of most concern. Placement of a reflectance mode sensor on the less active anterior trunk would likely encounter much less motion artifact.
Physiologically, with premature newborn infants, two SpO2 sensors are needed to track the transition from fetal to air-breathing blood circulation pathways. The most suitable locations for these sensors would be the right upper anterior chest (pre-ductal), and the left lower anterior abdomen (post-ductal). The two sensors would also, optimally, use the same center wavelengths of LED light and be pre-calibrated to be as equivalent as possible. Sensor placement on the chest and abdomen would also minimize the time delay between a central change in blood oxygenation and the detection of the change by the sensors.
Unfortunately, that still leaves tissue oxygen metabolism, specifically detection of excess tissue oxygen delivery, and the resulting vital organ vascular and tissue injury, without coverage. Even in adult medicine and surgery, oxidative-stress injury remains an extremely important, but still-unresolved root cause of ischemia/reperfusion injuries (IRI) associated with stroke, heart attack, and organ transplant. Premature infants uniquely suffer devastating oxidative-stress injuries to the microvasculature of the retinas of the eyes, the brain, and the gut. Tissue hyperoxia cannot be detected by pulse oximetry, which only detects the oxygen saturation of the arterial blood. Prevention of IRI apparently needs the ability to monitor for, and prevent, delivery of relatively too much oxygen to vital organ microvasculature.
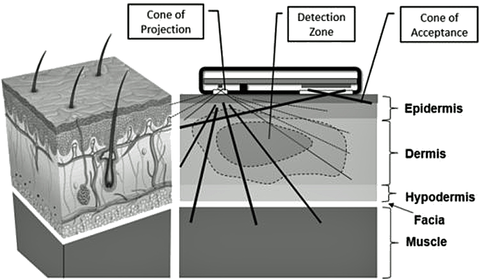
Careful study and some thought about the photonic design of existing sensors identified a possible design problem with the prior art. The opportunity to build and test a possible solution to this problem presented itself in 1999 and, with the assistance of Excalibur Engineering, LLC, Logan, Utah, a series of engineering prototype sensors were built and bench tested. Hypoxemia challenge tests, where the subject briefly breathed nitrogen gas, showed the new sensor to have a very robust and apparently accurate SpO2 response in comparison with simultaneous data from a medical SpO2 sensor. However, the LabVIEW graphing revealed unexpected responses in the raw sensor data that could not be explained by published spectral responses due to blood oxygen saturation.
This unexpected response phenomenon has recently been confirmed and extended to explore the photonic response of skin tissue to briefly breathing extra oxygen, which resulted in the discovery of what appears to be a rapidly reactive, robust, and non-invasive means of detecting tissue hyperoxia that has not previously been reported or available in any form. Existing “tissue oxygen sensors” cannot determine that the current delivery of oxygen to the tissue is optimum for the tissue metabolism, as the present sensor appears to do. Further, our sensor’s response to briefly breathing nitrogen consistently returns to baseline as quickly as it develops.
However, the sensor’s signal response to a few breaths of oxygen persists for several hours; long past the return of a normal range SpO2 level. We interpret this latter photonic signal pattern as an indication that the biochemical reactions due to excess oxygen briefly delivered to the skin tissue are molecular-level oxidative injuries due to reactive oxygen species (ROS), which require several hours to repair before returning to the baseline that existed prior to the excess oxygen exposure. Thus, we believe this recently discovered photonic skin tissue hyperoxia signal response is the key response that needs to be avoided in many critical medical applications, such as care of premature infants, and during resuscitation and care of IRI-prone conditions in patients of all ages and sizes.
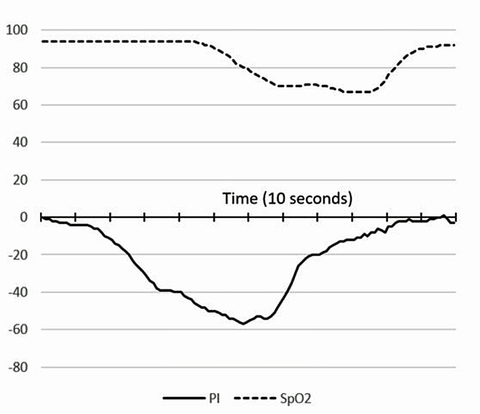
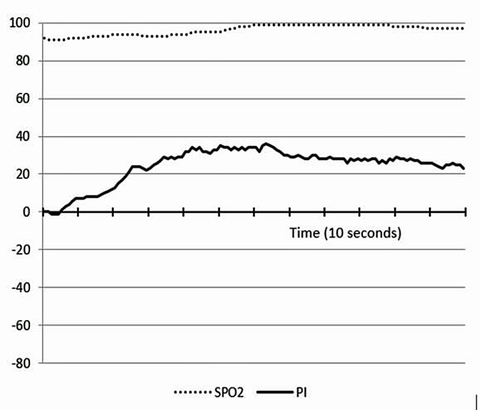
The robust and complex pattern of our new sensor’s response to physical exercise has also been very interesting and informative. This response apparently shows both the current physiologic cost of the athlete’s effort, and a unique signal baseline change over time that appears to correlate with the onset of fatigue during extended heavy exercise.
Typically, the initial photonic signal response during warm-up is that the skin quickly becomes relatively hypoxic (i.e. anaerobic), like when nitrogen gas is briefly inhaled, as arterial perfusion of the skin is reflexively diverted to supply muscles and more vital organs. However, as exercise continues, there is a photonic signal trend toward the pattern seen with skin tissue hyperoxia, as when excess oxygen is inhaled; even though the SpO2 remains constant throughout the exercise period. Since these signal responses occur despite unchanging SpO2 monitor values, we believe this signal response during exercise indicates dynamic regulation of energy conversion chemistry in the skin.
The trend during extended exercise can also be interpreted as a regulated adaptation of antioxidant activity in skin tissue, in response to the physiologic stress of decreased perfusion induced by exercise. This new data has been reviewed by several professional trainers and world-class elite athletes, who strongly concur that this new Physiology Index (PI) information is more functionally relevant than heart rate and heart rate variability in assessing the quality of exercise and recovery regimens.
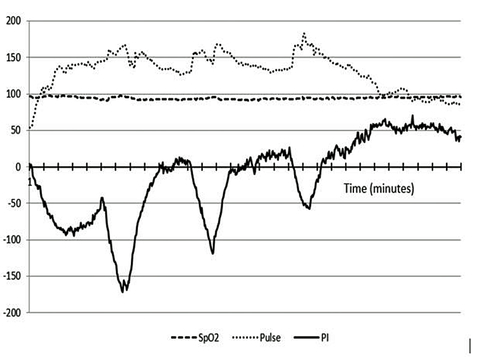
We believe the remarkable new insights obtained by monitoring athletes may be logically equated to the stresses encountered by premature infants during labor and delivery. Our sensor shows that the skin tissue of healthy adult athletes immediately becomes strongly hyperoxic upon stopping extended heavy exercise, when the skin is re-perfused by full flow of their normal oxygen saturation arterial blood. It is, therefore, reasonable to assume that a similar down-regulation of tissue antioxidant activity is occurring in the entire body of a fetus during labor-induced stress that is severe enough to result in decreases in heart rate!
This labor-induced distress is very likely to result in survival response adaptations to enable tolerance of lower oxygen delivery. However, this adaptation also may set the stage for potentially devastating IRI-like injuries to vulnerable organs, including the eyes, brain, and gut, when the newborn’s blood oxygen is quickly raised above the oxygen delivery tolerance of vulnerable tissues.
With the current lack of a biosensor capable of detecting the occurrence of tissue hyperoxia, clinicians only have pulse oximetry, adapted from adult medical care, to guide the infant’s arterial oxygen saturation into a “normal newborn” range. Current medical literature clearly shows that, especially for premature infants, less than about 30 weeks gestation, there remains a major, only partially controllable, risk of oxidative stress injury using current technology and methods.
Conclusion
Our current business path is to initially develop a reference design wireless wearable sensor module to be worn in an armband on the upper arm. We will then license the sensor technology to manufacturers of sports monitoring systems, because the entry barrier to this market is less severe than the regulated medical device market. However, we believe this new biosensor technology ultimately needs to be integrated with existing vital function monitoring sensor systems to provide key new information leading to reducing complications of premature birth.
Further, there is the potential that this sensor would be useful in reducing or preventing IRI, such as is currently a significant problem during reperfusion following ischemic stroke and heart attack, and during transplant organ implantation. We anticipate it will also be useful in generally improving neuroprotection during surgical anesthesia, and during resuscitation following cardiac and/or respiratory arrest in patients of all ages.
About the author
Guy M. Hatch, M.D. practiced as a Board Certified General Pediatrician for 21 years in Logan, Utah. He then led an R&D team in successful development of a reflectance SpO2 sensor for use with premature infants. During testing of this sensor, a previously unreported photonic phenomenon was discovered. For the following eleven years, he was a Mechanical Design Drafter at ATK, Logan, Utah. He is currently a co-founder and the CTO of Reveal Biosensors, Inc., San Jose, CA, continuing the development of a new biosensor based on his earlier discovery. He can be reached at: [email protected] and (435) 757-8081.